Glucocorticoid-induced tumor necrosis factor receptor family-related protein (GITR) is a critical regulatory molecule in modulation of T cell immune responses. Academician GAO Fu’s group report the mouse GITR (mGITR) and mGITR ligand (mGITRL) complex structure and find that the binding interface of mGITR and mGITRL is distinct from the typical tumor necrosis factor superfamily (TNFSF)/TNF receptor superfamily (TNFRSF) members. mGITR binds to its ligand with a single domain, whereas the binding interface on mGITRL is located on the side, which is distal from conserved binding sites of TNFSF molecules. Mutational analysis reveals that the binding interface of GITR/GITRL in humans is conserved with that in the mouse. Substitution of key interacting D93-I94-V95 (DIV) in mGITR with the corresponding K93-F94-S95 (KFS) in human GITR enables cross-recognition with human GITRL and cross-activation of receptor signaling. The findings of this study substantially expand the understanding of the interaction of TNFSF/TNFRSF superfamily molecules and can benefit the future design of biologics by targeting GITR/GITRL.
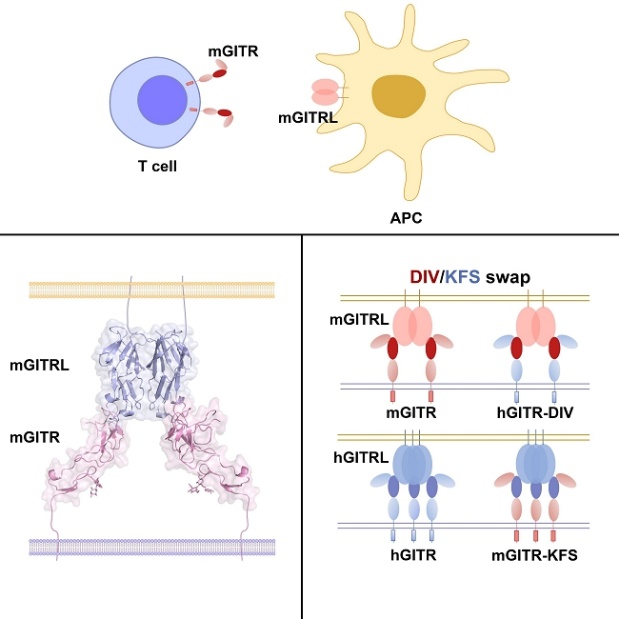
Structure of the GITR/GITRL complex and the DIV/KFS motif
Article access: https://www.cell.com/cell-reports/fulltext/S2211-1247(21)01183-9