Heavy metals' contamination of the aquatic environment is a major concern worldwide, especially lead (Pb) ion. The toxicity of heavy metal ions in water is mainly associated with their ready accumulation in the food chain. To date, the most often used sorbents for the separation of lead ions are carbon-based sorbents, organic and metal composite. Among them, organic material (e.g., Aminophosphonate) has been reported as efficient sorbent for lead recovery. These modifications may involve the grafting of reactive moieties onto titanium oxide, zirconium, and apatite. However, the two-step preparation of these composite adsorbents certainly increases the time and cost of the process.
Recently, the team cooperated with Professor Ibrahim El-Tantawy El Sayed of Menoufia University in Egypt to publish the paper "selective lead (II) absorption using aminophosphonate based sorbents: effect of amine linker, characterization and absorption performance". In this regard, to fulfill the research gap, the preparation of aminophosphonate derivatives by a one-pot synthesis strategy and its utilization for selective Pb(II) adsorption from aqueous media was effectively carried out. This work has innovatively been investigated with a deep understanding of waste hazards and metal recycling with a research background in environmental chemical engineering. In brief, this study focuses on synthesizing two new α-aminophosphonate-based sorbents using a one-pot synthesis procedure by direct reaction between amine precursors (e.g., ethylenediamine (DA) and diethylenetriamine (TA)) with salicylaldehyde and diphenyl phosphite. This specific contribution brings complementary information on the effect of the length of the amine chain on the sorption properties in terms of sorption criteria (uptake kinetics and sorption isotherms) as well as on separation criteria. The simple and environmentally-friendly alternative method (involving ionic liquid as an eco-friendly catalyst instead of a hazardous one) is another breaking contribution. Associated with an extensive characterization of materials, those conjunctions of studies contribute to a complete and innovative approach. The physicochemical structure and properties are first characterized by elemental analysis, the result demonstrated that the maximum sorption efficiency is influenced by the length of the chain, and reached 0.694 mmol Pb g-1 for DA and 0.494 mmol Pb g-1 for TA. The sorption isotherms are modeled by the Sips equation for DA (and the Langmuir Dual Site equation) and by the Langmuir equation for TA. Thermodynamic parameters (∆G°, ∆H°, and ∆S°) indicate the spontaneous, endothermic nature and randomness increases during the sorption process. The uptake kinetics (equilibrium reached ≈120 min) is almost equally fitted by the pseudo-first-order rate equation and the pseudo-second-order rate equation. To evaluate the selectivity of the sorbents for Pb(II), complementary experiments were performed using multi-component equimolar solutions (Co, Ni, Zn, Cd, in addition to Pb). The sorbents are efficiently recycled for at least 5 cycles using 0.2 M HCl as the eluent (loss in sorption and desorption performances ≈10-14% at the fifth cycle, compared with the first cycle). In the future, the prepared materials could be regarded as potential adsorbents for the efficient removal of Pb(II) from aqueous media.
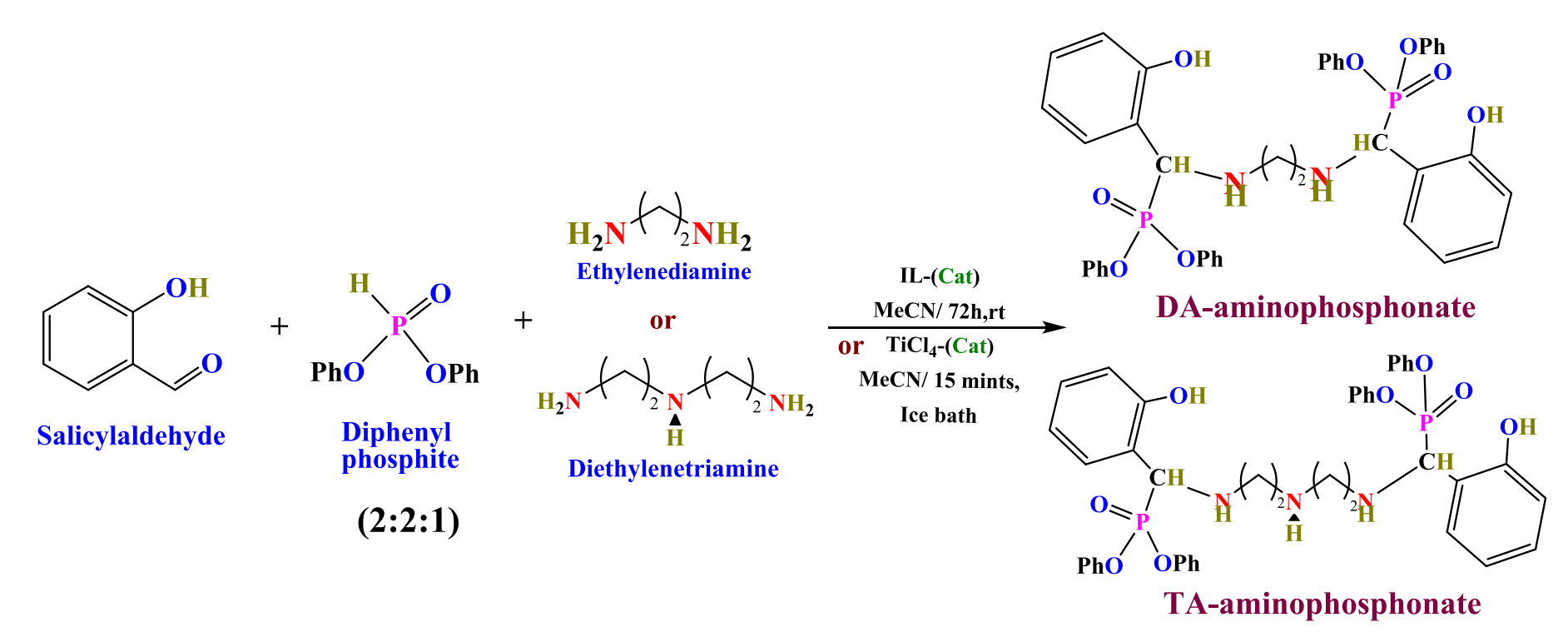
Scheme 1. Schematic route for the synthesis of α-aminophosphonate derivatives (DA and TA)
Article link:https://www.sciencedirect.com/science/article/pii/S1385894722017971